Latest News
Showing the 5 most recent news items. Scroll down to see archived posts.
The Challenges of Medical Device Development
When we are approached by an inventor with an idea for a new medical device, it’s our job to turn it into a commercial reality. However, the medical device sector is one of the world’s most highly regulated and rightly so. You cannot gamble with patient’s health. Get it wrong and the results can be fatal.
Working with a specialist medical device manufacturer can reduce costs, speed up your time to market and ensure high quality standards.
Read the full article at Health Tech World: The Challenges of Medical Device Development
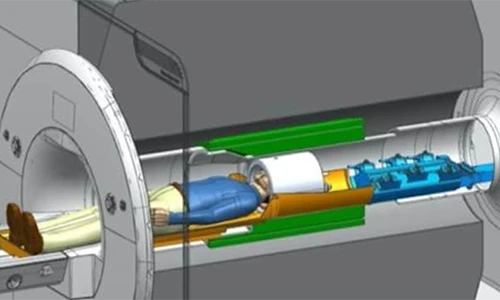
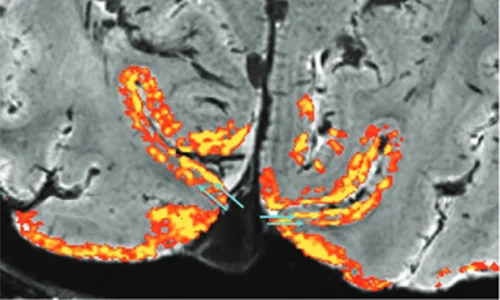
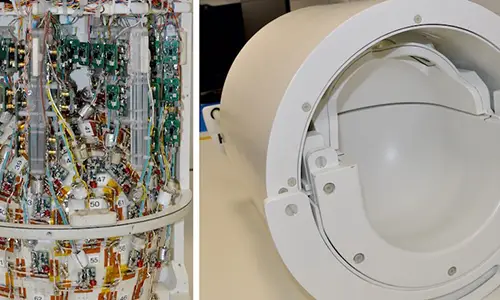
06 Apr 2024Glasgow Firms Behind Cutting-Edge Tech Examining Brain Disorders
Two Glasgow businesses are behind new brain scanning technology that could lead to ground-breaking research into brain disorders such as schizophrenia.
The next-generation MRI scanners are being developed for the University of California Berkeley and will allow doctors and scientists to see the brain in greater detail than ever before. Wideblue is a medical device product consultancy and fellow Glasgow business MR Coilech is a leader in high-density MRI head coil development
Read the full article: Glasgow Firms Behind Cutting-Edge Tech Examining Brain Disorders
15 Feb 2024Ensuring Your Device will be Ready for Market
Medical device development is a highly regulated sector and rightly so. Patient safety, comfort and welfare needs to be at the core of any medical device design. However, with a plethora of standards, regulations and certifications in this field it can be hard to navigate and ensure your device will be ready for market.
Read the full article on New Design: Ensuring Your Device Will be Ready for Market
09 Feb 2024Berkeley Develops New MRI Scanners with Wideblue and MR Coiltech
Advanced MRI scanners being developed by University of California Berkeley will allow doctors and scientists to see the brain in greater detail than ever before which could lead to treatments for brain disorders such as degenerative diseases, schizophrenia, and developmental disorders, including autism spectrum disorders.
Read the full article on Tech Today: Berkeley Develops New MRI Scanners with Wideblue and MR Coiltech
14 Dec 2023Revolutionising the Treatment of Respiratory Disease
As an award-winning, medical device developer, we are thrilled to have worked with TidalSense for the past 10 years to produce the world’s first handheld capnometer targeting the management of chronic respiratory conditions.
Read the full article at Design Products & Applications Magazine.
06 Dec 2023Archive
2023
- Berkeley Develops New MRI Scanners with Wideblue and MR Coiltech14 Dec 2023
- Revolutionising the Treatment of Respiratory Disease06 Dec 2023
- Bringing Your Medical Device to Market18 Sep 2023
- Scottish Companies to Help Develop Satellite Communication System08 Sep 2023
- Technology Scotland: Member Spotlight22 Aug 2023
- Breakthrough Magazine: Science Dream Made Real22 Jun 2023
- Medical Device Design: Getting it Right the First Time05 Jun 2023
- Glasgow Engineering Innovation to Boost Medical Imaging24 Apr 2023
- Fraunhofer Partners to Develop Optical Communications System22 Mar 2023
- Preparing the Perfect Prototype20 Mar 2023
- newdesign, 2023 Yearbook: Preparing the Perfect Prototype23 Feb 2023
2022
- Early Detection Technology for Volcanic Activity Takes Another Stride Forward with Scottish Design Firm20 Dec 2022
- Wideblue and University of Glasgow Give Volcano Eruption a Wee-G Touch19 Dec 2022
- Wideblue and A2E to Showcase Cutting-edge Production D&D at CENSIS 202228 Sep 2022
- Wideblue Ranked Among Top Glasgow Based Medical Device Companies27 Sep 2022
- Scottish Product Design Firms Fire Up Jobs Drive Thanks to New Client Wins20 Sep 2022
- Scottish Product Design Firms Start Recruitment Drive13 Sep 2022
- Pivot Makes Smart Move to Help Scottish Start-ups01 Aug 2022
- Newest Member of The Association of British HealthTech Industries (ABHI)14 Jul 2022
- Pivot UK a Sponsor of Filament STAC14 Jul 2022
- CENSIS Tech Summit and Conference14 Jul 2022
- Companies Collaborate to Develop Novel Patient Transfer Device07 Jul 2022
- Wideblue to Develop Novel Transfer Device10 Jun 2022
- BuffaloGrid’s Charging Tech Empowers Struggling Communities and Refugees19 Apr 2022
- Livingston Product Design Firm Celebrates 20th Anniversary with Trio of Key Hires21 Mar 2022
- A2E Gears Up For 20th Anniversary With Appointment of Three New Key Hires18 Mar 2022
2021
- Glasgow University and Wideblue Partner to Detect Volcanic Eruptions16 Dec 2021
- The Herald: Last Founding Manager at Wideblue to Step Down06 Dec 2021
- Phone Charger for Developing World01 Nov 2021
- The Journey to a Sustainable Future28 Oct 2021
- Product Design Scotland Toolkit: Scaling Manufacturing and Managing Supply Chains25 Oct 2021
- The Scotsman: A2E Adding Jobs After Recent Business Wins28 Sep 2021
- Forbes: Computer Chip Shortage Crisis Shows Supply Chains at Risk19 Jul 2021
- Wideblue Managing Director, Russell Overend, was the subject of this week’s Herald newspapers Monday Business Interview.24 May 2021
- 41 Action News: Effects of Chip Shortage, How to Avoid Impacts13 May 2021
- The Insider UK: Glasgow Design Agency Joins Consortium07 May 2021
- Bdaily News: Making Technology Happen for 15 Years06 Apr 2021
- The Insider UK: Scottish Tech Companies Partner to Revolutionise the Atomic Clock06 Apr 2021
- newdesign – Yearbook 2021: Wideblue – making technology happen for 15 years18 Mar 2021
- The Insider UK: Scottish tech firms join forces on ground-breaking MRI scanner20 Jan 2021
- The Herald: Two Glasgow Firms Hail ‘Ground-Breaking’ New MRI Scanner19 Jan 2021
- First Trial of “ground-breaking” MRI Scanner Project Completes14 Jan 2021
- Wideblue Contributes to Ground Breaking MRI Scanner Project14 Jan 2021
2020
- Wideblue’s Customer MR CoilTech Successfully Trials New 96 channel 7 Tesla MRI Head Coil21 Dec 2020
- Wideblue named as a winner in German Product Design Awards 202110 Nov 2020
- Wideblue Earns Int’l Acclaim for CRiL23 Sep 2020
- How We Pivoted Production to Tackle Covid-1921 Sep 2020
- Breath-Measuring Device in Upcoming NHS Trials Could Monitor Recovery from Covid-1901 Jul 2020
- Breath-measuring device in upcoming NHS trials could monitor recovery from Covid-1901 Jul 2020
- Responding to COVID-1912 May 2020
- Revolutionary New Crop Camera will Boost Global Agriculture29 Apr 2020
- Product Developer’s Big Break for Lung Patients14 Mar 2020
- Wideblue in the Daily Express11 Mar 2020
- Coronavirus Update10 Mar 2020
- Wideblue’s Camera to Make Crop Disease Detection Easier25 Feb 2020
- Wideblue medical devices on show at California Trade Show14 Feb 2020
- Wideblue and academic partners publish paper on a new Hyperspectral camera10 Feb 2020
2019
- Wideblue Building Infrared Medical Devices for Clinical Trials12 Dec 2019
- Wideblue Wows at Med-Tech Innovation Expo21 May 2019
- Wideblue presenting new product designs at Med Tech Innovation Expo, NEC Birmingham 15-16th May03 May 2019
- Wideblue visit the Offshore Renewable Energy (ORE) Catapult’s National Renewable Energy Centre31 Mar 2019
- Wideblue sponsor Doctor Of Engineering in Applied Photonics15 Mar 2019
2018
- Wideblue client Polaroid Originals featured in major article in the Guardian20 Sep 2018
- Product Design Specialist WideBlue Acquired by Kansas Suitor20 Apr 2018
- Wideblue and Pivot announce merger19 Apr 2018
- Wideblue led team completes Hyperspectral Camera project17 Apr 2018
- Pivot International Acquires Wideblue10 Apr 2018
- Congratulations to Park Mains High School in getting through to the UK finals of the school 4×4 challenge23 Feb 2018
- Wideblue, Fraunhofer and Mologic announce new development project for antibiotic susceptibility test for UTIs20 Feb 2018
2017
- Wideblue, together with partners GSS and CRiL, win Electra European Electronics Industry Award 201708 Dec 2017
- Wideblue demonstrate the HCC Hyperspectral Crop Camera and the Peek Retina mobile phone ophthalmoscope02 Nov 2017
- Wideblue upgrades ISO9001 and ISO13485 to new standards15 Sep 2017
- Wideblue engineered Calcivis dental imaging system presented to parliamentary group24 Jul 2017
- Wideblue video released – Backing the Game Changers01 Jun 2017
- Wideblue client Impossible joins with Polaroid12 May 2017
- Wideblue win double Platinum Product Design Award for Peek Retina17 Mar 2017
- Wideblue named as finalist in Knowledge Exchange Awards03 Feb 2017
- Wideblue will be showing a case study at the forthcoming UWS Health Technologies and Medical Devices Showcase20 Jan 2017
2016
- Wideblue clients reach finals of 2017 Scottish Life Science awards16 Dec 2016
- Offshore Wind Sensors – Technology Demonstration02 Dec 2016
- Wideblue Director Grant King to speak at the first Technology Scotland design workshop in Edinburgh on 1st November.28 Oct 2016
- Wideblue to demonstrate three new products at CENSIS Technology Summit30 Sep 2016
- Wideblue customer CRiL completes clinical trials with great results delivered from NTC129 Sep 2016
- Wideblue deliver batch of prototype ophthalmoscopes to Peek12 Sep 2016
- Wideblue start new wind turbine blade optical monitoring project01 Sep 2016
- Wideblue wins new Hyperspectral Crop Camera Project01 Jul 2016
- Wideblue client The Impossible Company launches the Wideblue engineered I-1 camera11 Apr 2016
- Wideblue director Grant King to speak at Bio Dundee Conference16 Mar 2016
- Wideblue demonstrate new capnometer products to Princess Anne29 Feb 2016
- Wideblue customer CRiL start clinical trials at Addenbrookes hospital with new NTC1 product15 Feb 2016
- Wideblue complete build of CRiL new NTC1 medical devices for clinical trials25 Jan 2016
- Wideblue complete Solid State Optical Respiration Rate Capnometer (SOSORC) project on time and on budget11 Jan 2016
2015
- Wideblue Customer CRiL Achieve ISO 1348509 Nov 2015
- Wideblue finalists at British Engineering Excellence Awards29 Oct 2015
- Wideblue announced as finalists in British Engineering Excellence Awards01 Sep 2015
- Wideblue achieve accreditation to ISO9001 and ISO1348501 Aug 2015
- Wideblue Purchase Thermal Camera for Project Work18 May 2015
- Wideblue Start New Project for 3D Remote Visual Inspection in the Nuclear Industry24 Apr 2015
- Wideblue Client Anacail Secures £2 Million in Equity Funding22 Apr 2015
- Wideblue Presenting at Innovate UK Meeting “Creating Smart Products from Smart Materials”17 Mar 2015
- Wideblue to implement ISO13485 and ISO9000 by mid 201515 Jan 2015
- Wideblue Completes Innovate UK project on Cryopreservation of Therapeutic Cells Project13 Jan 2015
- Wideblue Customer CRIL Moves into Wideblue Offices During Product Development Stage08 Jan 2015
- Wideblue Client Ingenious Audio Launches Crowdfunding Initiative on Kickstarter.com05 Jan 2015
2014
- Wideblue and Partners Win SBRI Phase 2 Competition for COPD Medical Device10 Dec 2014
- Wideblue Client Peek Vision launch Crowdfunding initiative on Indegogo05 Dec 2014
- Wideblue Starts SBRI Medicines Adherence Project31 Oct 2014
- Wideblue director Grant King presented Product Development workshop at St Andrews University01 Aug 2014
- Wideblue Director in Commonwealth Games Queen’s Baton Relay17 Jul 2014
- Wideblue Visits Potential Chinese Manufacturers01 May 2014
- Wideblue Signs Contracts with the IMPOSSIBLE Film Company01 Apr 2014
- Wideblue Presents Case Study at the Encompass Event04 Mar 2014
- Wideblue Moves to The West of Scotland Science Park28 Feb 2014
- Wideblue Client Calcivis granted a CE Mark in Europe12 Feb 2014
- Wideblue wins new TSB Photonics for Health Competition17 Jan 2014
2011
- Life Science Firm wins Funding Grant06 Dec 2011
- SKF Launch Top of the Range Laser Alignment System01 Nov 2011
- Contactspod makes contact with the travel and leisure industry in Cannes25 Oct 2011
- Wideblue exhibiting at Convergent Technologies Showcase and Conference06 May 2011
- Meet Wideblue at the Scottish Technology Showcase19 Apr 2011
- Wideblue client wins Innovation Award21 Mar 2011
- Human tissue testing in medical and diagnostic device development23 Feb 2011
- Supporting medical device engineering students and graduates23 Feb 2011
- Understanding the product opportunity03 Feb 2011
2010
- Computer Simulations in Bioengineering seminar – “Optical Analysis of Ambulight PDT”21 Dec 2010
- Wideblue to exhibit at Technology World 201001 Nov 2010
- Wideblue supports new UKTI investment forum21 Oct 2010
- Wideblue CEO judging the Converge Challenge – fostering Scotland’s entrepreneurial talent pool16 Sep 2010
- Design Council case study demonstrates the value of Wideblue design to university technology transfer projects15 Sep 2010
- Wideblue invited to join international electronics consortium06 Sep 2010
- Wideblue design saves money during development of new drugs01 Jul 2010
- Wideblue exhibiting at this years Scottish Technology Showcase26 May 2010
- Driving Scotland’s Healthcare Innovation26 May 2010
- Wideblue presents workshop at Technology Changing Healthcare event19 May 2010
- Web launch for Kelvin Vision Multifocal Imager14 May 2010
- Centeo Launches First Product15 Feb 2010
- Wideblue supports the John Logie Baird Awards for Innovation27 Jan 2010
2009
- Wideblue Director presents a case study at the Life Sciences Scotland Med tech Forum11 Nov 2009
- Wideblue founding partner in Kelvin Capital syndicate07 Oct 2009
- Starting a business: What the over-50s need to know26 Sep 2009
- PWB Health Product Available at Boots04 Sep 2009
- Wideblue Exhibits at major UK Medical Device Technology event10 Jun 2009
- Scottish Parliament congratulates PWB Health on design award win29 May 2009
- Breastlight wins Excellence in Design Gold Award09 Apr 2009
- The Nexxt Big Thing in Women’s Health18 Feb 2009
- Wideblue Exhibits at ITI Members’ Meeting16 Feb 2009
- Wideblue to Raise Investment Levels07 Jan 2009
2008
- Wideblue Wins Top Innovation Award22 Sep 2008
- Wideblue Supports the John Logie Baird Awards Programme 200818 Aug 2008
- Scottish Government Minister John Swinney Visits Wideblue30 Jul 2008
- West Dunbartonshire MP John McFall Visits Wideblue06 Jul 2008
- Wideblue Working with Dragon’s Den Winners to Provide Clean Drinking Water for the Developing World16 Mar 2008
- Wideblue Spinout, PWB Health Ltd, named Most Promising Life Sciences Company08 Feb 2008
- Wideblue Announces New Tech Investment Partnership18 Jan 2008